Introducing CHARM
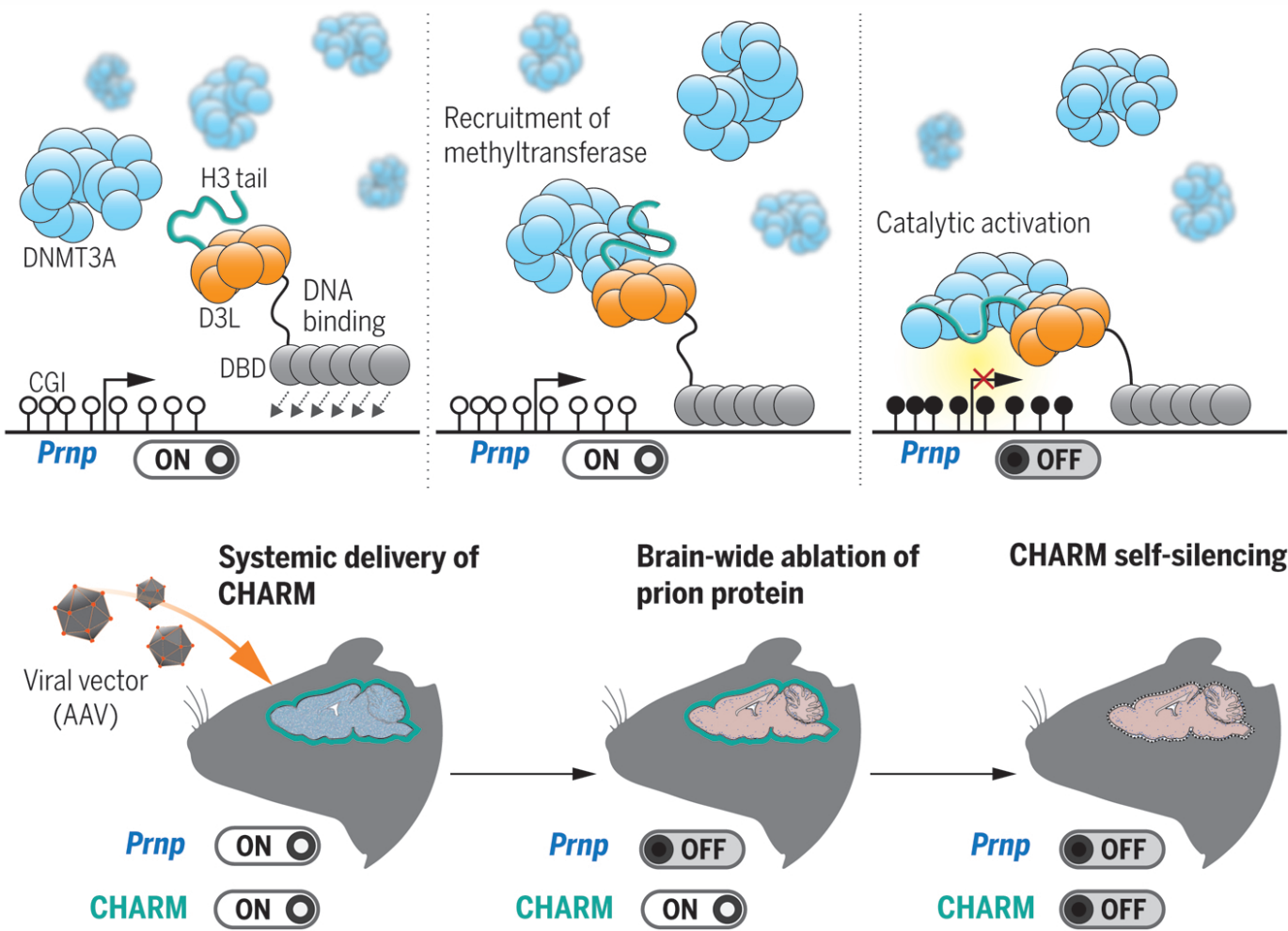
Today we announce the development of CHARM, a new epigenome editor designed to methylate DNA, which we show can silence PrP in the whole mouse brain [Neumann & Bertozzi 2024].
This new invention comes from our collaboration with Jonathan Weissman’s lab at the Whitehead Institute next door to Broad. The project was generously funded by NIH through the Somatic Cell Gene Editing (SCGE) consortium. Dr. Weissman’s work has appeared on this blog before — he made some major contributions to the understanding of the prion mechanism in yeast years ago. Today, much of his lab is focused on technology development to manipulate the epigenome — the code that decides which genes are turned on or off in our cells. When we were applying for SCGE funding in 2022, we approached him to see if he would be willing to partner with us on methods to silence PrP. His lab had recently published CRISPRoff [Nunez 2021], an approach that uses DNA methylation to turn genes off. Jonathan agreed that silencing PrP could be a perfect test case for new technologies that people in his lab were working on.
For background on DNA methylation, see last week’s primer. In short: DNA methylation is when cytosine or “C” in your DNA gets a methyl group added, making it 5-methylcytosine (5mC). This doesn’t change the sequence of the DNA per se, since 5mC still pairs with G just like C pairs with G, but it has profound effects on the use of the DNA. Methylation appears to directly repel transcription factors, the machinery that decides which genes get transcribed into RNA, and methylation also makes the DNA crumple up tightly (“chromatin compaction”) and acquire a host of other changes that cause genes to be turned off. Compared to other epigenomic changes, DNA methylation is thought to be quite permanent: our cells mostly use it for silencing genes they will never want to turn on again.
Edwin Neumann, a grad student in the Weissman lab, had distilled 20 years of literature on DNA methylation into a vision for a new tool, now called CHARM. CRISPRoff includes a DNA methylation enzyme to turn DNA off, but it can cause too much methylation, leading to toxicity. The Weissman lab had tried ways of utilizing the cell’s existing DNA methylation machinery instead, but one problem was that even if you could direct that machinery to the place you wanted it, the enzyme was “off” by default. Edwin realized you needed three elements: you needed to grab onto our cells’ existing DNA methylation machinery, tell it where to go, and turn it on. To tell it where to go, you need a protein domain that sequence-specifically binds DNA, so that you can target a gene of interest. This DNA-binding domain could be CRISPR-Cas9, a TALE, or a zinc finger (ZF). (Note that Sangamo is working on a different application of ZFs to silence PrP by a different mechanism, and our lab is collaborating with them, but they were not involved in the CHARM study). This DNA-binding domain is then fused to two pieces of protein machinery called D3L and a unmethylated histone H3 tail, separated by a linker. The DNA-binding domain guides this fusion protein to the DNA at the gene of interest, D3L grabs onto an enzyme called D3A that can methylate DNA but which is normally turned off, and then the histone tail turns that enzyme on. The result is that, only at the gene of interest, you get a profound increase in DNA methylation, causing the gene to be silenced. Edwin had to tune and optimize every piece of this machine needs to get it just right. In fact, it’s scary to think that one could have easily missed it altogether: for instance, only one of the linkers that Edwin tested yielded appreciable activity.
When the DNA-binding domain is a TALE or a ZF, the whole CHARM package is small enough to fit inside an AAV. AAV is a viral vector used to deliver genes to the brain. In this study, we used PHP.eB, which is an AAV vector that delivers genes beautifully to the mouse brain after a single IV injection, but which would never work in humans because it doesn’t have a human receptor. But many teams worldwide are working to engineer AAVs that will work in humans, and as I blogged last month, our collaborator Ben Deverman at the Broad has just announced a major breakthrough in engineering AAVs to bind human receptors, making the possibility of such a gene therapy for humans no longer science fiction. To demonstrate feasibility of using CHARM in an AAV to silence PrP, we used a version of CHARM with a published ZF as the DNA-binding domain.
When we treated mice with AAV packaging CHARM, we saw an increase in DNA methylation at the PrP promoter and a profound decrease in PrP RNA and protein levels in whole mouse brain hemispheres:
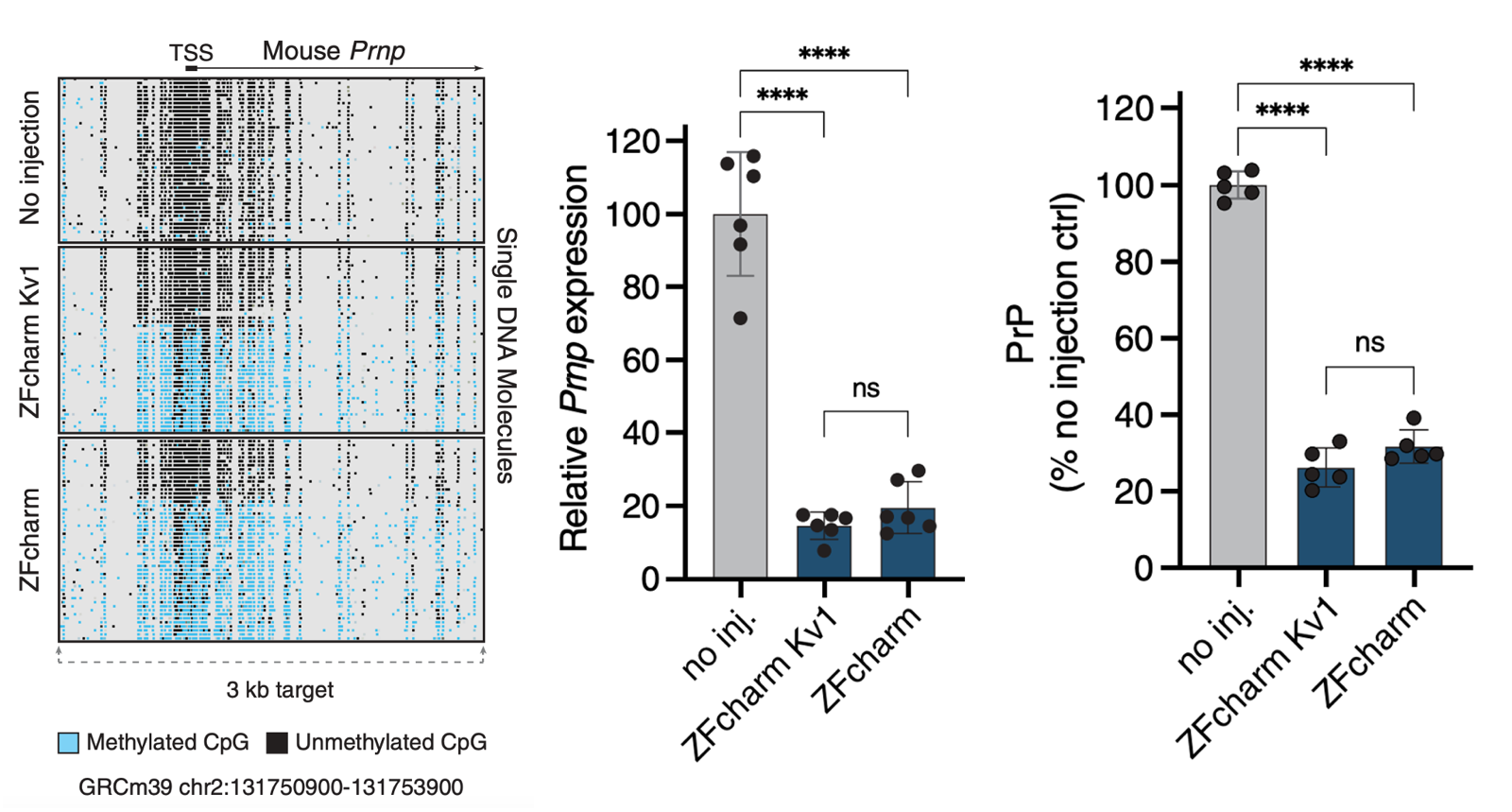
Figure 4B, C, D from [Neumann & Bertozzi 2024]. Data from C57BL/6N mice treated with 1.5e13 vg/kg AAV PHP.eB; RNA and protein data are from whole brain hemispheres.
AAV is quite long-lasting, with some reports indicating that a gene packaged in AAV can be produced for 10 years in humans, while there are also reports indicating expression can be lost [Muhuri 2022]. Ideally you don’t want to count on continuous expression of the AAV, in case it is lost at some point. You may also not want to produce an AAV transgene continuously, in case it proves immunogenic, or in case your editor accumulates more and more off-target effects over the years. Yet, at the same time, AAV is fairly likely to stick around a good while — it’s not like an lipid nanoparticle delivered to the liver, where it’s here today and gone tomorrow. How to use a potentially long-lasting AAV to make a change that could be both longer-lasting than the AAV itself, and that doesn’t stress the system by keeping a transgene around longer than you need to? To address this question, Tessa Bertozzi from the Weissman lab developed a self-silencing approach: you include a piece of the target DNA sequence in the AAV construct itself, so that once CHARM is done silencing the target gene, it also silences itself. The DNA methylation change is expected to be permanent (we show up to 13 weeks durability in mice in this study), but the expression of AAV does not need to be permanent.
To be clear, it’s still a long road to any clinical application of CHARM. Consider the timeline on which PrP-lowering ASOs have been developed. We first met with Ionis to the project 2014, we obtained positive data in mice and announced the collaboration in 2018, and the ASO clinical trial didn’t launch until the end of 2023. We should always strive to move faster than that, while also remembering that biology is hard and developing drugs is hard, and any number of obstacles could crop up.
Long though the road ahead may be, we are absolutely stoked about CHARM. Above all, the demonstration of a one-time gene therapy for prion disease is really exciting, especially because advances in AAV engineering finally make it imaginable to achieve in humans what we achieved here in mice. Our first email to Jonathan Weissman, reaching out to propose a collaboration, was sent only 2 years and 1 month ago. This project has raced past all my expectations. I take home two lessons from this: one about people, and one about biology.
On people: this project succeeded because of specialization and teamwork. Each person, and each lab, was able to focus on what they excelled at. That may sound obvious, and maybe in industry it is, but in academia it’s too rare. To be sure, it’s not to say that no one tried new approaches or learned new things: for instance, it was Edwin’s first time doing Nanopore sequencing to examine methylation marks, and it our team’s first time doing retro-orbital injections. But we saved enormous amounts of time by having a team with diverse skill sets, where no one had to waste time reinventing wheels, getting other people’s methods to work “in our hands”. Edwin, with support from Elaine Wu, worked incredibly quickly through different versions of CHARM to find the right linker, the right histone tail, the right set of protein domains to stitch together to yield a potent whole. Tessa designed the self-silencing approach as well as performing the flow experiments and figuring out how to get the in situ RNA imaging to work. The Deverman lab produced virus, batch after batch, on tight turnaround and with stringent quality control. The Vallabh/Minikel lab handled the animal experiments, tissue processing, and PrP quantification. Within our lab, again, we had specialization, focus, and speed: Fiona Serack single-mindedly led the in vivo experiments on a stringent timeline, with Nikita Kamath assisting with assays, with Alissa Coffey as project manager organizing everything, and we also got to lean on deep animal experience from Michael Howard, our comparative medicine lead. Now imagine the alternate reality where one lab (or worse, one postdoc), had needed to not only invent CHARM but also learn to make virus, inject animals, and run ELISAs. We could have easily taken 5 or 7 years to accomplish what we learned here in 2 — or more likely, never gotten there at all. Critically, though, while each of us had our own skillset and responsibilities, we were all oriented around the shared goal. Fiona recently opined that what makes this project “magical” is that no one ever feels like they are asking for favors, or waiting for someone whose priorities lie elsewhere. We are all “all in” on this effort. Efficiency requires us each to be specialists, yet a team needs to cohere enough to act as, in the aggregate, a generalist. A mission can be so clarifying. Still, a mission alone is not enough: one also needs the resources to fight for that mission. Here, it was possible for all three labs to fully dedicate their time and people to this shared project because the NIH funded it through a very special, translationally-oriented U19 mechanism. For this we need to thank Tim Lavaute, PJ Brooks, Chris Boshoff, and others at NIH who had the vision to dedicate these funds not just to doing cool science, but to racing forward as quickly as possible towards things that could really be drugs.
On biology: the success with CHARM is also, to me, a reminder that biology is vast. There are a lot of potential solutions to any given problem, and we should never despair. If something we had barely imagined 2 years ago can already look this good, imagine what we can achieve in our lifetimes.
